Abstract
Introduction: We have previously reported the results of cohort A from a single arm, phase II clinical trial of lenalidomide with rituximab (R2) as frontline treatment for patients with previously untreated follicular lymphoma (FL), Fowler N et al Lancet Oncology 2014. Recent randomized studies (RELEVANCE) did not demonstrate superiority of either R2 or R-Chemo in untreated, high GELF FL, but follow up is short. We now report outcomes of an additional extended dosing cohort (12 mo of R2) and the long term follow up of the both dosing schedules in untreated FL.
Methods: A total of 154 pts were included in the original clinical trial (FL, n=80; MZL, n=31; SLL, n=43). Characteristics were collected at the time of starting R2 treatment. Patients received lenalidomide 20 mg/day on days 1-21 of each 28-day cycle and rituximab 375 mg/m2 on day 1 of each cycle (6 cycles; schedule A) and lenalidomide 20 mg/day on days 1-21 of each 28-day cycle for cycles 1-6 then lenalidomide 10 mg/day on days 2-22 for cycles 7-12 with rituximab 375mg/m2 IV x1 weekly on cycle 1 and day 1 of every subsequent cycle (12 cycles; schedule B). Responders continued treatment for at least 6 but up to 12 cycles. The primary endpoint was overall response rate (ORR); secondary endpoints were complete and partial response (CR, PR), safety, and progression free survival (PFS). PFS was defined as time from starting treatment to disease progression or death, event free survival included time from starting treatment to discontinuation due to any cause and overall survival (OS) was defined from the time of initial diagnosis of FL to death/last follow up.
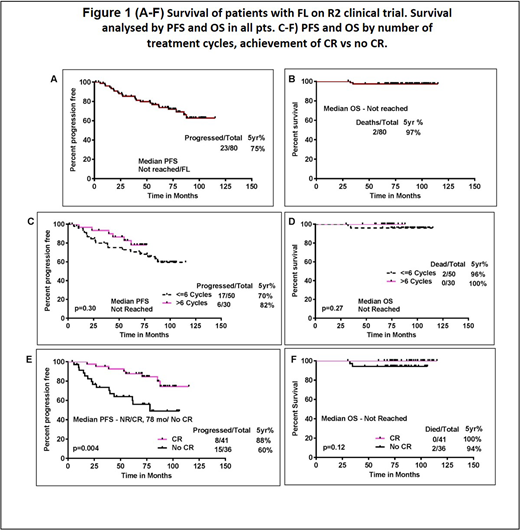
Results: Eighty pts with FL were enrolled in study and followed a median of 86 months. Median age was 58 years (range, 29 to 84); 50% were males. 61% pts had grade 1 FL and 39% had grade 2 FL. Schedule A was administered in 50 pts and schedule B in 30 pts. Seventy seven pts were evaluable for initial response assessment and 76 (98%) responded. The best response rate was 95% (87% CR/CRu). At the time of last follow up, 23 patients experienced disease progression, 13 lost to follow up (all had CR as best response and had completed tx), 4 came off study due to pt choice/financial and 4 due to intolerance (2 arterio-thrombotic event, 1 respiratory failure, 1 intolerance) during therapy. After a median follow up of 86 mo, 23 pts (29%) progressed, 5 yr PFS was 75%. Five yr PFS was 70% and 82% for pts on cohort A vs B respectively (P=.30). Overall, 2 pts died, with a 5 year survival 97%, Figure-1 (A-B). The median event free survival in pts with FL was 85 months with a 5 year EFS of 59%. Subgroup analysis showed no statistically significant difference in PFS with FLIPI score, bulky disease and by initial bone marrow involvement. Pts who achieved CR had significantly longer PFS compared to those who did not achieve CR (not reached vs 78 months; p = 0.004), however the OS was not significantly different between the two groups Figure-1 (C-F). Grade 3 or 4 hematologic AEs included neutropenia (28%), thrombocytopenia (3%), and no anemia. Count recovery occurred in all pts with follow up and/or dose modification. Nine pts developed second primary cancers, including one melanoma in-situ, 3 localized skin cancers, and 2 secondary hematologic malignancies.
Conclusions: A combination of lenalidomide with rituximab produced durable responses in pts with FL. At a follow up of 7 years, the majority of pts remain in remission and patients who achieved CR had the best outcomes. Five year PFS may be longer in pts who received 12mo of therapy, but will need larger analysis to confirm. Further studies are ongoing to analyze mutation dynamics and genomic profile to identify molecular biomarkers.
Fowler:Janssen: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding. Nastoupil:Novartis: Honoraria; Juno: Honoraria; Gilead: Honoraria; TG Therappeutics: Research Funding; Spectrum: Honoraria; Janssen: Research Funding; Merck: Honoraria, Research Funding; Karus: Research Funding; Genentech: Honoraria, Research Funding; Celgene: Honoraria, Research Funding. Westin:Apotex: Membership on an entity's Board of Directors or advisory committees; Celgen: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals Corporation: Membership on an entity's Board of Directors or advisory committees. Wang:Kite Pharma: Research Funding; Novartis: Research Funding; Pharmacyclics: Honoraria, Research Funding; Dava Oncology: Honoraria; Juno: Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MoreHealth: Consultancy; Acerta Pharma: Honoraria, Research Funding. Samaniego:ADC Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal